Contact Us
Receive a timely response to an enquiry about a service, process or technical questions.
Speak to us
040 -29886224

Clinpacifics life science solutions Medical and Regulatory Consulting team provides comprehensive pre-clinical support, regulatory affairs assistance, and expert medical and pharmacovigilance consulting services.
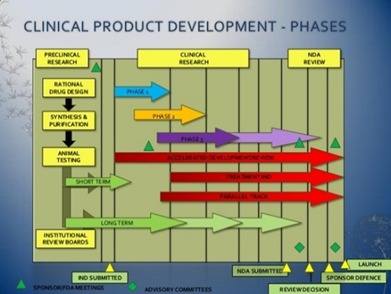
Drug Development Strategies
Choosing the right development strategy is essential for achieving commercial success. As biotech companies invest in advanced therapies and global trials, there is growing emphasis on regulatory planning, including the design of clinical protocols, nonclinical testing, and manufacturing processes. Greenline Clinical Research offers top-tier regulatory services to accelerate drug development in the complex and ever-changing research landscape.
Core Regulatory Services
Core Medical Services
From the initial stages through development and approval, our team supports you throughout every phase of the process.
Receive a timely response to an enquiry about a service, process or technical questions.
040 -29886224